Anode is Positive or Negative
Since electrons carry a negative charge then the anode is negatively charged. In the general sense current refers to any movement of electrical charge.
Anode and cathode are two terms that are often used interchangeably with positive and negative in batteries.

. The negative electrode is called the cathode while the positive electrode is called the anode. Anode is negative in electrochemical cell because it has a negative potential with respect to the solution while anode is positive in electrolytic cell because it is connected to positive terminal. Think of a large parallel capacitor.
However in some experiments such as Gel Electrophoresis the anode is positive. The terms anode cathode positive and negative are not synonymous they can sometimes be confused which can lead to errors. In a galvanic voltaic cell the anode is considered negative and the cathode is considered positive.
The cathode of a device is the terminal where current flows out. This seems reasonable as the anode is the source of electrons and. Advertisement In a galvanic cell electrons will move in to the anode.
What is anode in xray tube. Cathode and Anode Remember charge can flow either from positive to negative or from negative to positive. The purpose of this article is to clarify and.
Answer 1 of 2. The anode by definition is the electrode where electricity. The anode and cathode are defined by the flow of current.
The label anode or cathode is not defined with respect to the electrostatic sign as explained in the post by Maurice. Link This is a common misconception. However you should keep in mind the.
Connect one terminal of. This is what happens during electrolysis. By current we mean.
In a battery or other source of direct current the anode is the negative terminal but in a passive load it is the positive. Is anode positive or negative in xray. The electrode where oxidation occurs in an electrochemical cell.
For goodness sakes man that answer is incomplete. However in an electrolytic cell the anode is taken to be positive whil. The anode is usually considered to be negative.
In a galvanic voltaic cell the anode is considered negative and the cathode is considered positive. This means the cathode is negatively charged the anode is positively charged. The anode of a device is the terminal where current flows in from outside.
Anode the terminal or electrode from which electrons leave a system. This seems reasonable as the anode is the source of electrons and. It is the positive electrode in an electrolytic cell.
Why is an anode negative and a cathode positive. Positively charged ions move to the. A terminal is not classified base on whether its positive or negative.

Electrophoresis Chemistry Anode Cathode Diagram Chemistry Respiratory Therapy Study Materials

Anodes And Cathodes Positive And Negative Bar Chart Mcat
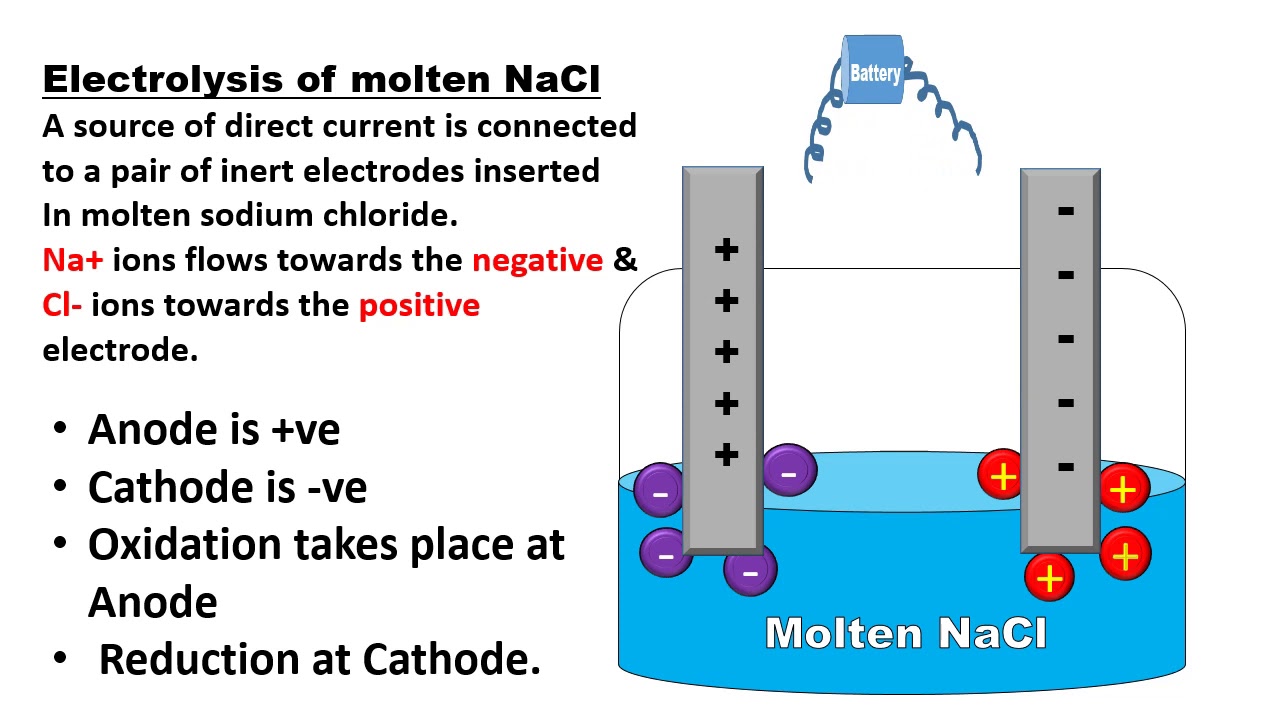
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

How To Find Anode And Cathode In Diode Diode Black Rings Tutorial

Comments
Post a Comment